 Society
Society

There have been some public concerns and pronounced hesitancy against the Chinese vaccine, despite the fact that WHO and Vietnamese health authorities have approved Sinopharm vaccine for emergency use.
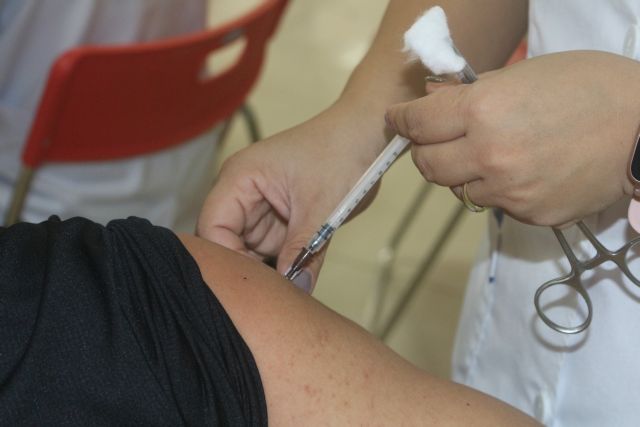
|
| Many residents of Móng Cái City in the northern province of Quảng Ninh received second doses of Sinopharm COVID-19 vaccines between August 5-12. — VNA/VNS Photo Thanh Vân |
HÀ NỘI — HCM City’s health department has issued official responses to the health ministry’s request for clarification of its deal for and use of the donation of five million doses of Sinopharm COVID-19 vaccine (Vero Cell).
The first shipment of one million doses arrived at Tân Sơn Nhất International Airport in HCM City on July 31 but the city has not yet rolled out the Chinese vaccines pending quality controls, vice chairman of HCM City’s People Committee Dương Đức told a press briefing on August 3.
The administration of the vaccines would be free and on a voluntary basis, he added.
Việt Nam to date relies mostly on Western vaccines – AstraZeneca, Pfizer and Moderna – in its vaccination drive, and there have been some public concerns and pronounced hesitancy against the Chinese vaccine, despite the fact that WHO and Vietnamese health authorities have approved Sinopharm vaccine for emergency use.
Two days after the press briefing in HCM City, Hải Phòng’s People’s Committee on August 5 officially requested the health ministry and HCM City to borrow 500,000 doses to give to priority groups including long-distance drivers and workers in China-invested companies and other volunteers as the allocation to the northeastern city so far has not matched its needs. The move is interpreted as Hải Phòng is willing to take the Chinese vaccines off HCM City’s hands if they are not used, and likely prompted the health ministry’s request for clarification on HCM City’s part.
The health department’s document noted that with the sponsorship of Vạn Thịnh Phát Group, a private conglomerate founded by a Vietnamese national of Chinese origin in HCM City, the State-controlled Saigon Pharmaceutical Company (Sapharco) in HCM City has successfully negotiated and signed a contract to purchase Vero Cell vaccine with China National Biotec Group Company Limited (CNBG), China Sinopharm International Corporation, Beijing Bio-Institute of Biological Products Co Ltd. and Sinopharm International Hong Kong Co Ltd.
Vạn Thịnh Phát supported the price negotiations and covered the entire vaccine purchases along with related costs – tax, insurance fees, importing costs into Việt Nam, preservation and delivery of the five million doses from storage to vaccination points, with no strings attached, according to HCM City’s health department.
Sapharco is responsible for supplying contract content and details of payment costs related to the purchase, transportation and import of vaccines to HCM City People's Committee and the Ministry of Health upon request.
Regarding the use of the vaccine, the Vạn Thịnh Phát Group hands the entire shipments to the HCM City’s authorities to administer to the residents under their jurisdiction, according to HCM City’s health department. The health department is in charge of building plans on vaccine distribution, reception of the vaccines, storage, allocation and administration of the vaccines for people free of charge in line with health ministry’s guidelines.
The health department noted that amid the COVID-19 developments and vaccine shortage, the city could consider diverting Vero Cell vaccines to other localities if agreements with Vạn Thịnh Phát are reached.
HCM City is the first locality in Việt Nam to have imported COVID-19 vaccines – other than sources from the health ministry – after receiving permission from Deputy Prime Minister Vũ Đức Đam.
Sapharco is one of the 36 companies with the capacity and permission to import vaccines, according to the health ministry.
Sinopharm vaccines as part of the 500,000 doses donated from the Chinese Government in late June have been administered in many localities of Việt Nam, especially for Chinese nationals in Việt Nam, northern border regions and those who want to go to China for business or study. — VNS