 Society
Society

The Hà Nội Medical University on Sunday kicked off the first phase clinical trial of the ARCT-154 vaccine against COVID-19 with 100 volunteers from Hà Nội.
|
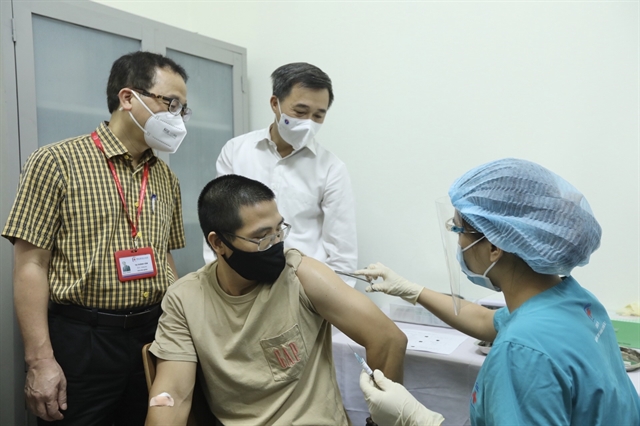
| Deputy Minister of Health Trần Văn Thuấn (standing, first right) witnesses the ARCT-154 vaccine injection for a volunteer. — VNA/VNS Photo Minh Quyết |
HÀ NỘI — The Hà Nội Medical University on Sunday started the phase 1 of clinical trial of the mRNA COVID-19 vaccine named ARCT-154 vaccine with 100 volunteers from Hà Nội.
The self-amplifying mRNA vaccine is developed by the US-based Arcturus Therapeutics who partners with VinGroup's VinBioCare for support in clinical trials and manufacturing (at a facility in Hoà Lạc Hi-tech Park, Hà Nội).
Professor Trần Văn Thuấn, Deputy Minister of Health, head of the health ministry's special working group on clinical trial research and development of COVID-19 vaccines, expressed his joy when witnessing the first injection of the experimental for volunteers.
"This vaccine is being tested at the Hà Nội Medical University, and we strongly believe and look forward to completing the research soon to promote domestic vaccine production," he said.
He adds that the health ministry hopes that the research process will complete its third phase by the end of the year, and Việt Nam could have more autonomy in vaccine supplies amid global shortage.
The technology allows a lower dose of vaccine, while the immune stimulation lasts longer, for a quick and simple prevention of the COVID-19, and is capable of fighting dangerous new variants of coronavirus such as Alpha, Beta, Delta and Gamma.
"I believe that, under the direction of the Government and the Prime Minister, with the help of domestic and international experts, the clinical trial of the vaccine will soon be successful and Việt Nam will soon become self-sufficient in vaccines against COVID-19," said Thuấn.
He also highly appreciated the careful preparation, enthusiasm, dedication and responsibility of scientists from the Hà Nội Medical University and other facilities participating in clinical trials.
The deputy minister said this was the third vaccine following the Nanocovax, developed by Nanogen in HCM City, which is in phase 3 trials, and Covivax vaccines, currently in phase 2, in the series of research and technology transfers under the direction of the Government.
The health official thanked scientists for accompanying and working with the ministry to gradually complete the research and development of vaccines in the country. He also thanked volunteers who contributed to the clinical trial of the vaccines.
Professor Tạ Thành Văn, chairman of the Hà Nội Medical University’s council, the main researcher of the ARCT-154 vaccine clinical trial, said, "When testing the Covivac vaccine, the research team had to rely on the press to publish information about recruiting volunteers widely for several days to have enough people. But for the ARCT-154 vaccine, after only two days of announcing the recruitment of volunteers, we received more than 800 people. Through screening, more than 100 qualified people have been selected.”
It is expected that the administration of the first 100 doses will be completed by the end of Monday.
As the first person to receive this vaccine, a man named T. from Hà Nội expressed his pleasure to contribute to scientific research so that Việt Nam can soon have more domestic vaccines.
Another volunteer, H., 36 years old, working in logistics in Đống Đa District, went to the university early in the morning to have his health examined again before the injection.
"I came here to both dedicate myself to scientific research and serve my personal vaccination needs," said H.
Head of the Hà Nội Medical University said that the clinical trial process was carried out in accordance with the outline plan approved by the health ministry on August 2.
Before the injection, the volunteers were screened and tested.
Volunteers will receive the second doses of ARCT-154 vaccine in four weeks.
In phase 2,300 volunteers will be involved, while in phase 3, up to 20,600 volunteers are expected to be participating in the trials. — VNS




