Society
Society

The Vietnamese health ministry has recently issued guidelines on protocols for diagnosis and treatment of rare brain/abdomen blood clots in the setting of low levels of blood platelets occurring after COVID-19 vaccinations.
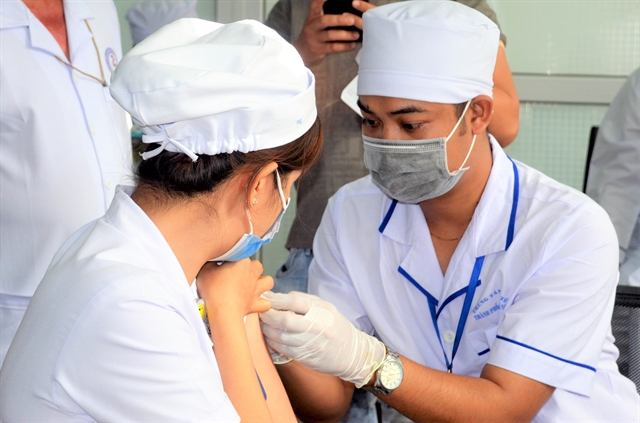
|
| Medical staff at Sóc Trăng General Hospital in the southern province of Sóc Trăng receive COVID-19 vaccines on Monday. — VNA/VNS Photo Trung Hiếu |
HÀ NỘI — The Vietnamese health ministry has recently issued guidelines on protocols for diagnosis and treatment of rare brain/abdomen blood clots in the setting of low levels of blood platelets occurring after COVID-19 vaccinations.
It stressed that these incidents are serious, but a very rare and unusual complication that seems to occur more among women younger than 60 years old.
The incidents that take place after administering COVID-19 vaccines by AstraZeneca and Johnson & Johnson were documented by drug authorities and vaccine safety monitoring agencies in many countries, the ministry noted.
The clinical symptoms – including lasting serious headaches, seizures, breathing difficulties and chest pains, abdominal pains, pain and oedema of the lower extremities, but rarely bleeding or skin haemorrhage or internal bleeding – show within four to 28 days after being administered the vaccine.
Imaging diagnostics – such as ultrasound and pulse doppler – of sites with clinical manifestations such as abdomen or the limbs can show thrombosis. X-raying and computed tomography (CT) or magnetic resonance imaging (MRI) scans at suspected sites such as in the brain, lung, or painful areas etc. can show signs of thrombosis or bleeding.
The health ministry said that commune- and ward-level health stations and district-level medical centres must monitor people who have received the COVID-19 vaccines, and if the injected person shows any of clinical symptoms, medical staff must perform “emergency measures” and transfer the patient to the higher-level health facilities.
Health centres are also told to conduct a platelet count, imaging and X-rays on suspected patients to diagnose the underlying conditions, and contact experts for those with abnormal symptoms.
Vaccinated persons who suffer from severe headaches, localised neurological symptoms, seizures, difficulty breathing, chest pain, life-threatening bleeding should also be transferred to higher level.
Provincial- and municipal-level health facilities need to conduct tests and treatment of probable conditions that may be encountered in line with diagnosis and treatment guidelines of the Ministry of Health. If the patient’s condition progresses beyond the capacity of their diagnosis and treatment, it is necessary to consult a specialist or to refer to the higher-level medical facilities.
Central-level hospitals are supposed to receive the patients with severe reactions to vaccinations, and consult experts for treatment if necessary.

|
| An AstraZeneca COVID-19 vaccine shot is being prepared in Sơn Trà medical centre, Đà Nẵng City. — VNA/VNS Photo Văn Dũng |
Minister of Health Nguyễn Thanh Long issued Decision No. 1888/QĐ-BYT on April 15 to establish a Steering Committee for safe COVID-19 vaccination, which comprises of senior officials from the ministry and leaders of the ministry’s related departments along with Việt Nam’s leading experts and scientists in diverse fields from immunisation, infection, emergency resuscitation, intensive care, to haematology, cardiology and neurology.
The Steering Committee is tasked with guiding medical establishments in performing screening, monitoring and handling of adverse events following COVID-19 vaccinations and promptly assist localities in handling all situations safely.
Đào Xuân Cơ, Chair of Việt Nam National Association of Emergency, Intensive Care Medicine and Clinical Toxicology, and Deputy Director of Bạch Mai Hospital, the largest first-tier public hospital in the northern region, said that post-vaccination blood clot incidents could be handled just like any other commonly occurred blood clot.
Cơ reassured that the public could have “peace of mind” for receiving the COVID-19 vaccine given the very small risk, and even if such an incident does happen, the expertise of leading scientists would be available to intervene in a timely manner.
However, given the nature of the COVID-19 vaccine approved for emergency use, Việt Nam has been quite cautious in its vaccination drive.
“We do screening and counselling before the administration of the vaccine," Đào Xuân Cơ said.
"The injected person is monitored at the vaccination site for at least 30 minutes, then continues to be monitored at home for at least 24 hours and follow-up observations for another three weeks after injection. Hospitals are always ready to prevent severe reactions after injection.”
According to the Tuesday report of the National Expanded Programme on Immunisation, a record 50,104 people were inoculated on Monday.
Nearly 260,000 people – mostly frontline workers and medical staff – in 42 provinces and cities have been inoculated using AstraZeneca vaccines since the national vaccination drive began on March 8.
No rare blood clot incidents have been recorded so far, while the typical reactions (tiredness, mild fever, muscle pains, etc.) were reported in about 30 per cent of the vaccinated, even lower than the advisory from the manufacturer or European authorities’ reports.
The country currently has nearly 1 million doses of AstraZeneca vaccine (combined deliveries from the manufacturer and supplied via the COVAX Facility), along with 1,000 doses of Sputnik V vaccine as gift from Russia. — VNS