 Society
Society

Five people were given Nano Covax, the made-in-Việt Nam COVID-19 vaccine, at the highest dose on Tuesday during the ongoing human trials.
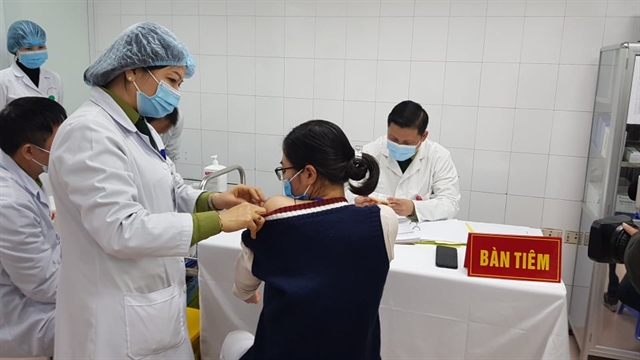
|
| A female volunteer was given the 75mcg dose of Nano Covax, the made-in-Việt Nam COVID-19 vaccine, on Tuesday morning at the Việt Nam Military Medical University in Hà Nội. — Photo from the Ministry of Health |
HÀ NỘI — Five people were given Nano Covax, the made-in-Việt Nam COVID-19 vaccine, at the highest dose on Tuesday during the ongoing human trials.
The Việt Nam Military Medical University in Hà Nội administered 75mcg Nano Covax shots to three female volunteers, and a further two volunteers were given the shot in the afternoon after 40 people previously received two lower dosages of 25mcg and 50mcg.
Developed and manufactured by HCM City-based Nanogen Pharmaceutical Biotechnology JSC, Nano Covax is being experimented with three types of dosage given in two shots 28 days apart.
Prior to human trials, the vaccine demonstrated its safety in white rats, hamsters, monkeys and rabbits.
Chử Văn Mến, director of the university’s Centre for Clinical Trials and Bioequivalence, said the volunteers receiving the jabs Tuesday morning were healthy females aged between 20 and 22.
He added that in the first phase, the selection of volunteers features a stringent layered process, and anyone who has previously reported allergies like rash or hives was not eligible.
One of the volunteers (whose name was protected) said she was a medical student and was fully informed of all of the risks of possible complications, so was not overly worried.
“To my knowledge, the first 40 volunteers’ physical health have all been okay so I felt at ease during the trial. And honestly, I am curious and want to know about the steps involved in the research of a vaccine,” a volunteer given the shot on Tuesday said.
The research team at the Việt Nam Military Medical University has kept in close contact with the volunteers but no abnormal signs have been recorded. The volunteers’ reactions to the shots – light fever and pain at the injection area in the arm – were expected and nothing to be concerned about, researchers said.
Volunteers
While there were more than 500 applicants for the vaccine trial, only about 200 actually showed up to take health examinations and 51 of them were selected for the first-phase clinical trial.
The research team is looking at the application documents again to reach the target of 60-65 volunteers, according to the military university’s official.
“The first phase trial is meant to assess the safety of the vaccine, which explains the stringent selection, in later phases of the trial, the criteria will be much more relaxed,” Mến said.
The first phase of the clinical trial for the vaccine, which typically involves a small group of subjects, is half completed, Mến said.
At this stage, the researchers can prepare to soon start the second phase of the trial which lasts for about 2-4 months, and the third phase which will last 3-6 months involving 10,000-30,000 volunteers.
The third phase will likely involve volunteers and take place in pandemic-hit regions of India, Indonesia, and/or Bangladesh, according to Nanogen.
It is expected that by the end of 2021, the full clinical data of the vaccine will be available for health authorities to evaluate before mass injections if everything goes according to plan.
All volunteers are covered under a special health insurance programme.
At the time of the trial commencement, Việt Nam was one of the 40 countries to be conducting human trials of COVID-19 vaccines. — VNS




