

Health departments throughout the country have been ordered to publish the winning bids for the supply of medical equipment to public hospitals.
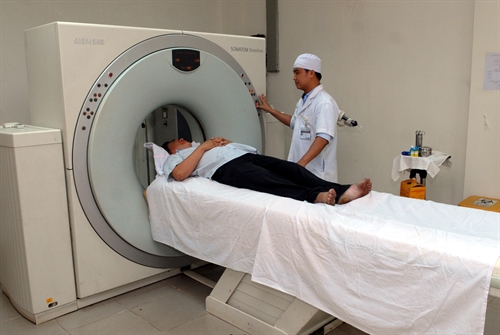 |
| A doctor at Xuân Lộc General Hospital in the southern province of Đồng Nai performs an MRI scan. Newly-issued decree on managing medical equipment went into force this month. – VNA/VNS Photo Mạnh Linh |
HÀ NỘI — Health departments throughout the country have been ordered to publish the winning bids for the supply of medical equipment to public hospitals.
The move aims to ensure consistent prices of medical equipment in the market, avoiding price hikes for the benefit of suppliers or hospitals, said Vũ Minh Tuấn, head of the Department of Medical Equipment and Construction under the Ministry of Health at a recent meeting in Hà Nội.
The meeting was designed to explain Decree No 36 about medical equipment management, that went into force this month, to health departments and companies supplying medical equipment.
Statistics from the Việt Nam Medical Equipment Association show that there are about 10,500 types of medical equipment in the market, 90 per cent of which is imported from countries like Japan and China. Most medical equipment used in health clinics is not checked and maintained periodically, according to the association.
Deputy Minister Nguyễn Viết Tiến said shortcomings were found in the management of medical equipment in hospitals, such as the use of substandard equipment or rare use of equipment, causing waste.
“Using substandard medical equipment is very dangerous for examining and treating patients,” he said.
Under the new decree, companies importing medical equipment must assume responsibility for its quality, preventing the smuggling of substandard equipment or medical equipment without clear origin, he said.
According to the decree, devices implanted into the human body must be tested in clinical trials on human beings before being used.
Only then can a device be issued a registration number, which would be revoked if the device was found to cause damage.
Tuấn, head of the Department of Medical Equipment and Construction, said the decree offered a stricter legal framework to get rid of companies supplying substandard medical equipment. — VNS




