 Society
Society

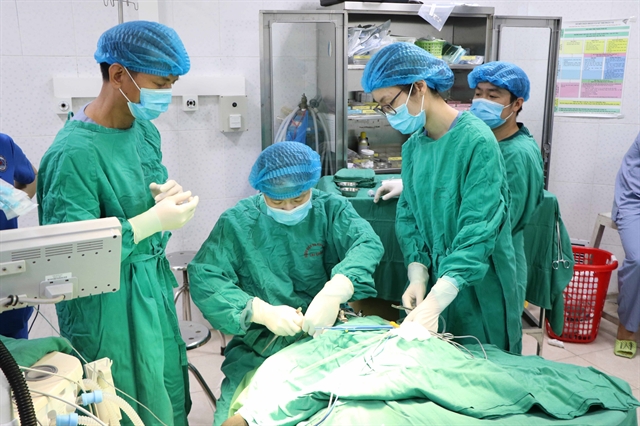 |
| Health facilities must also comply with principles on surgical safety and related checklists as specified by the MOH. — VNA/VNS Photo |
HÀ NỘI — The health ministry has urged medical facilities to improve their management and quality to raise patient safety and satisfaction, as well as minimise risks of medical incidents.
The official document is issued following inspections on patient quality and service quality conducted by the Department of Medical Service Administration under the Ministry of Health (MoH).
Hospitals under the MoH, provincial and municipal health departments, ministries and universities are required to take measures and be responsible for improving quality and safety at their facilities, preventing and classifying incidents and conducting reports per Circular 43/2018/TT-BYT.
They should also review and identify safety concerns that can affect patients and medical workers and promptly address them.
Other areas of focus include periodic reviews of technical procedures and standards, especially in classifying emergency cases, infection control as well as medication, equipment and supply management in emergency rooms, intensive care units and surgical departments.
Identified risks should be compiled into a mandatory incident reporting list for each facility, including the specifics for each medical speciality, procedure, department and unit function.
In the appendices to this list, the MoH requires that medical facilities accurately identify the patients to avoid confusion in providing services, prevent risks due to miscommunication, and regularly monitor medical records and consultation procedures as regulated.
Health facilities must also comply with principles on surgical safety and related checklists as specified in MoH Decision 7482/QĐ-BYT dated December 18, 2018.
Medical facilities must also conduct training on patient safety and enhance medical staff’s sense of responsibility, including new recruits and interns, in medical incident and risk reporting.
They should also raise awareness for cooperation among patients and their caretakers regarding this matter.
Inspection and supervision must be strengthened for periodic compliance reports on regulations on medical incident prevention and patient safety. Inspectors and supervisors are responsible for reporting to the unit’s management level.
In case of a medical incident, managers of the unit must immediately act upon receiving reports and allocate people in charge to contact and calm the patients and their caretakers while proposing appropriate solutions to the issues.
They must also provide support to medical practitioners involved in the incidents, avoiding emotional influence and premature conclusions on individual oversight without taking into account systematic errors.
The MoH also issues a guideline to differentiate between medication errors (ME) and adverse drug reactions (ADR).
Reviews should be conducted to detect common medication-related incidents in each stage, such as prescription, distribution, use and during monitoring period. — VNS